Tagged: science
Modeling Acids and Bases Lab
As my paradigm lab for Acids and Bases in Chemistry 2 we do a very simple classifying lab. My lab handout is here: Classifying Lab
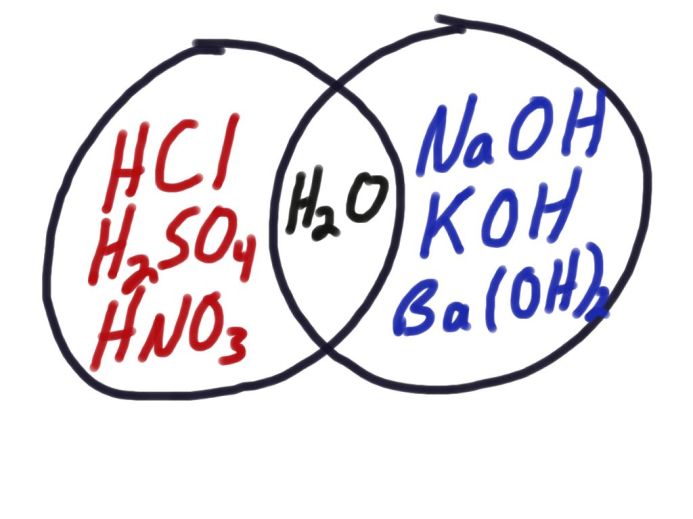
Basically what this entails is that I give the students 7 substances. HCl, H2SO4, HNO3, NaOH, KOH, Ba(OH)2, and H2O. They know the formulas and proper names, but NOT acid names (i.e. Nitric Acid). I have each group do some qualitative tests in well plates using various indicators and other chemicals. They take observation data in the table provided.
After all test are complete the students are to group the 7 substances into as many or few categories as they need. They just need to justify their groupings. After a little staring at the data most lab groups are able to see the pattern that emerges. They almost always create 3 groups. One contains HCl, H2SO4, HNO3, One has NaOH, KOH, Ba(OH) and H2O is by itself.
During our whiteboard discussion each group explains their reasons for putting certain things together. Sometimes they talk about only one indicator but most groups see the patterns emerge across all the tests. After we have an agreed upon grouping system I then pose the question, “Now look at your groups what do each of the substances have in common within a group?” Obviously you always get a variety of answers but the initial responses usually revolve around how one group has begins with Hydrogen and the other ends with Hydrogen. I remind them about what is significant about the order compounds are written in, and also what is significant about the Conductivity test we did. They realize we are dealing with ionic compounds, and the first ion is positive, while the second is negative. They also see the Hydrogen “on the end” always comes with an Oxygen, and we have hydroxide. Someone usually also realizes that water is really just HOH, thus a combination of both of these groups.
My class this year is quite bright and it didn’t take them long to verbalize these patterns. Within only a couple of seconds of this “light bulb” going off one young lady exclaimed. “Can we make this a Vin diagram!?” “Why not?” I said, “that would be a great way to model this!”
How perfect! Never a mention of the words Acid or Base, and we have Arrhenius’s Model! Concept before name!
Naturally the next day I challenged them by testing NH3.
To which a young man exclaimed. “Here we go again, we create a Model one day, and the next day we prove it wrong!”
I said, “Don’t shoot the messenger, that’s the way the world works.”
He then added, “in English they call it an exception to the rule, but in Science we create a new rule! That is why science rocks!”
Holy cow, have I done my job here? At least it made me feel good that I’m getting through to a few kids!